Collisions Ionic Bonding Game: Teaching Strategies to Use in Your Classroom
Have you been using the Collisions: Ionic Bonding Game with your students? Below are some additional strategies to help with planning your lessons.
- Before starting the game, ensure that students know the meaning of the following terms: positive charge, negative charge, cation, anion, net charge or total charge, ratio, neutrality.
- As students play, introduce the terms polyatomic ion and lattice structure.
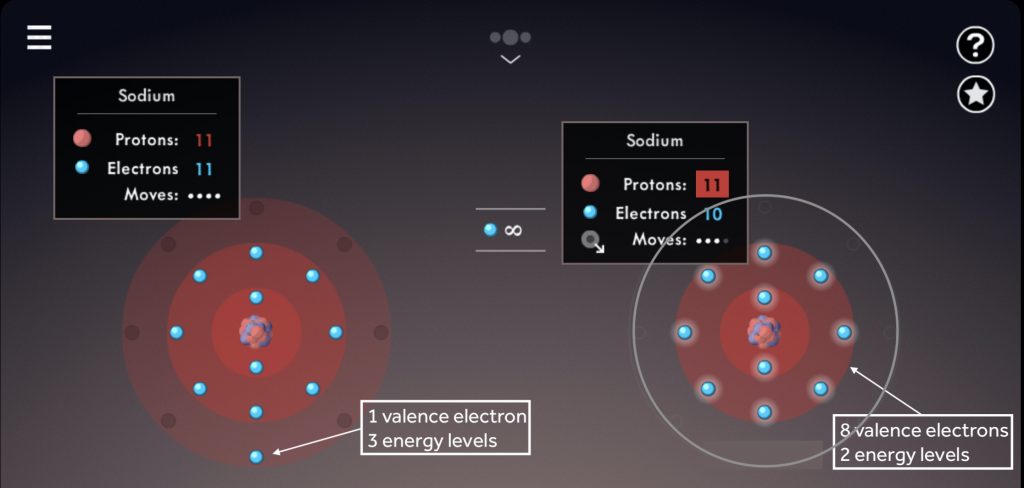
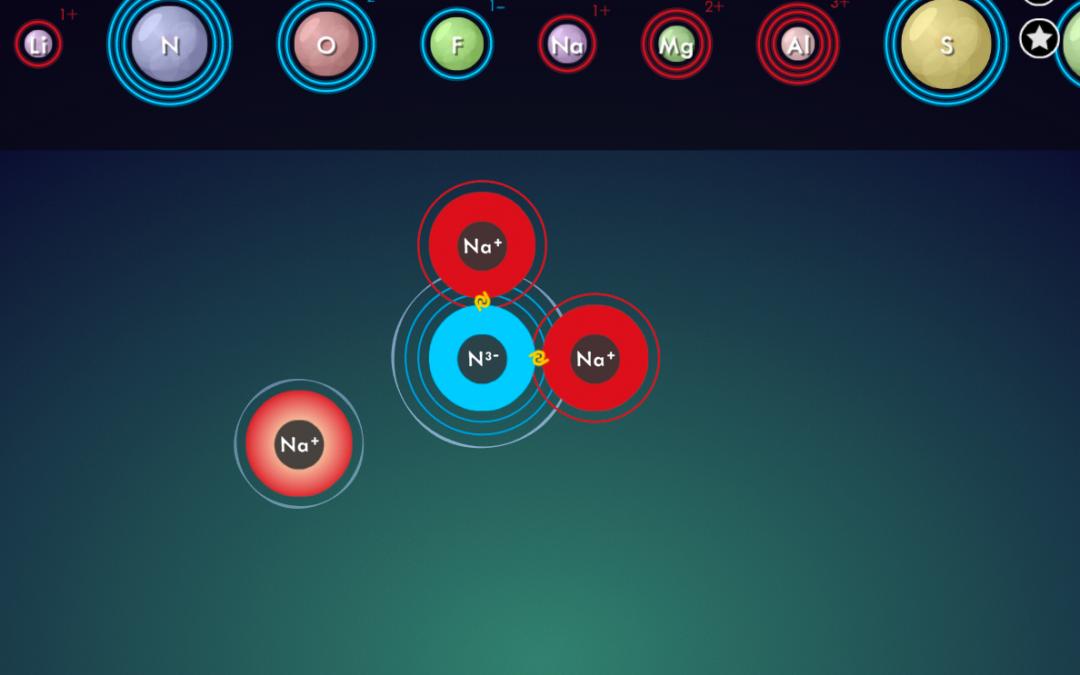
- Illustrate how to calculate the total cation charge, total anion charge, and explain how to ensure that those add up to zero in the end.
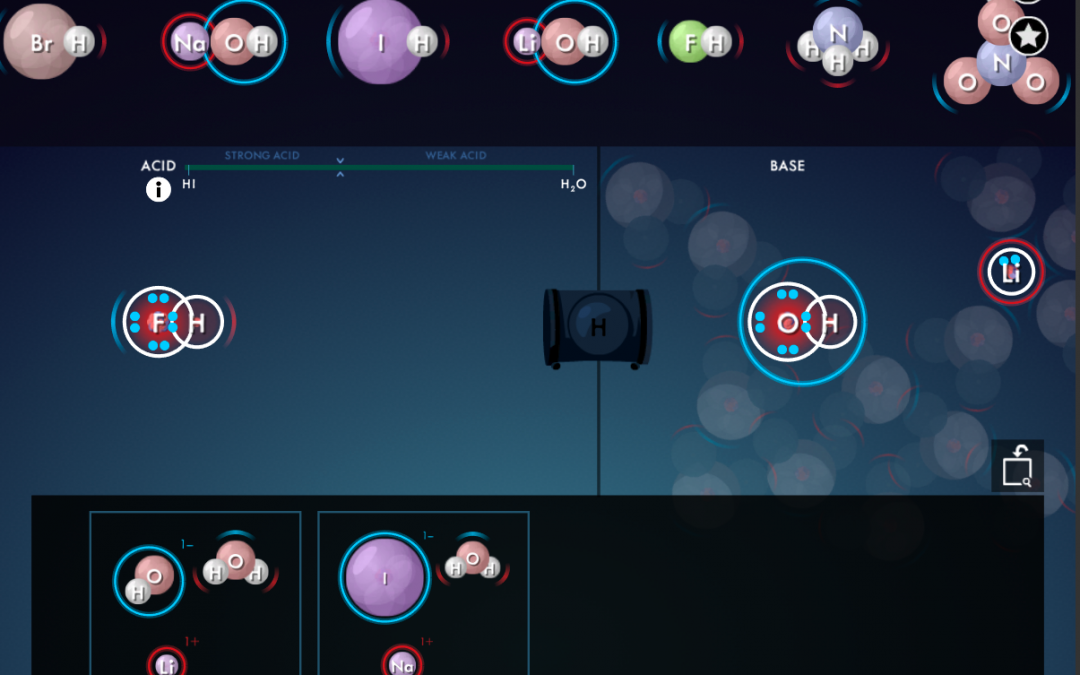
- Draw students’ attention to the “criss-cross method” that is often described on
- YouTube (also sometimes referred to as “swap and drop”), and point out the pitfalls of using it for compounds such as MgO, TiO₂ or Al₂(SO₄)₃.
- Explain electron transfer. Post these short Ionic Bonding videos for your students to watch!
- Challenge your students to master the Ionic Bonding Sandbox Achievements:
- Ionic compounds containing specific atoms
- Ionic compounds containing ions of specified charges
- Ionic compounds containing specific ions
- Ionic compounds from a given chemical formula
- Or, make up your own challenges and have students submit a screenshot of their work!
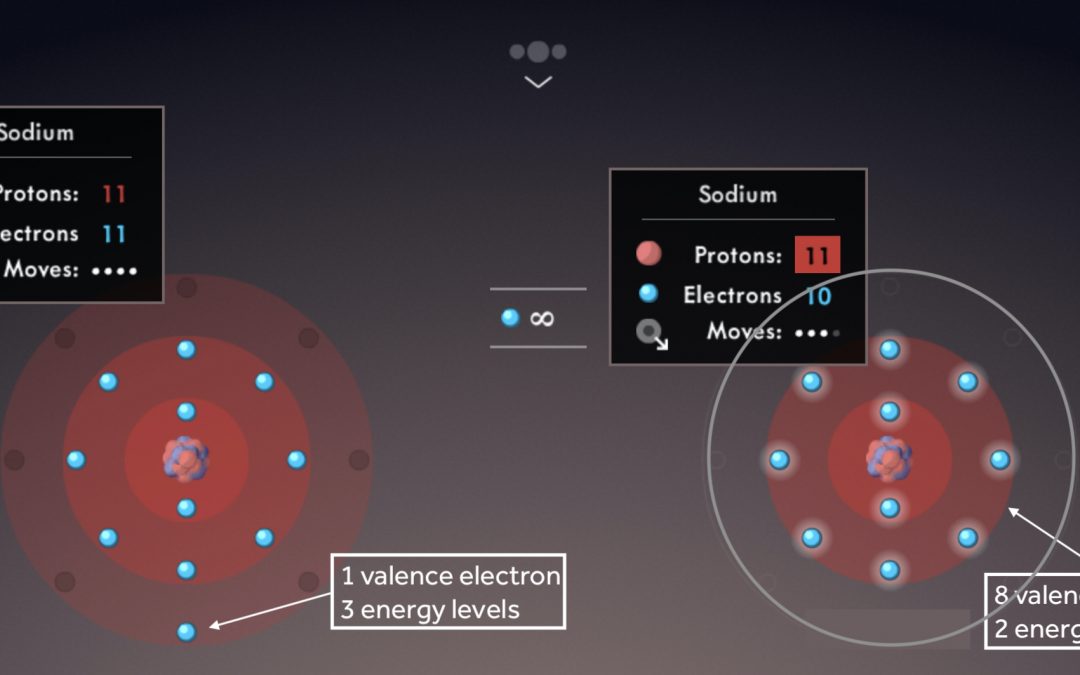
- Have your students identify ionization energy trends, and draw the trends as big arrows across each period and down each group on a blank periodic table.
- The Ionic Bonding game shares Connected Levels with the Ions game and the Acids & Bases game. Have your students complete both the Ions game and one of the other two games, to open up the pipe between these games and CONNECT their learning!