Have you been using the Collisions: Ions game with your students? Below are some additional strategies to help with planning your lessons.
Before starting the game, ensure that students know the meaning of the following terms: atom, proton, electron, ion
As students play, introduce the terms valence electrons, cation, anion, octet rule
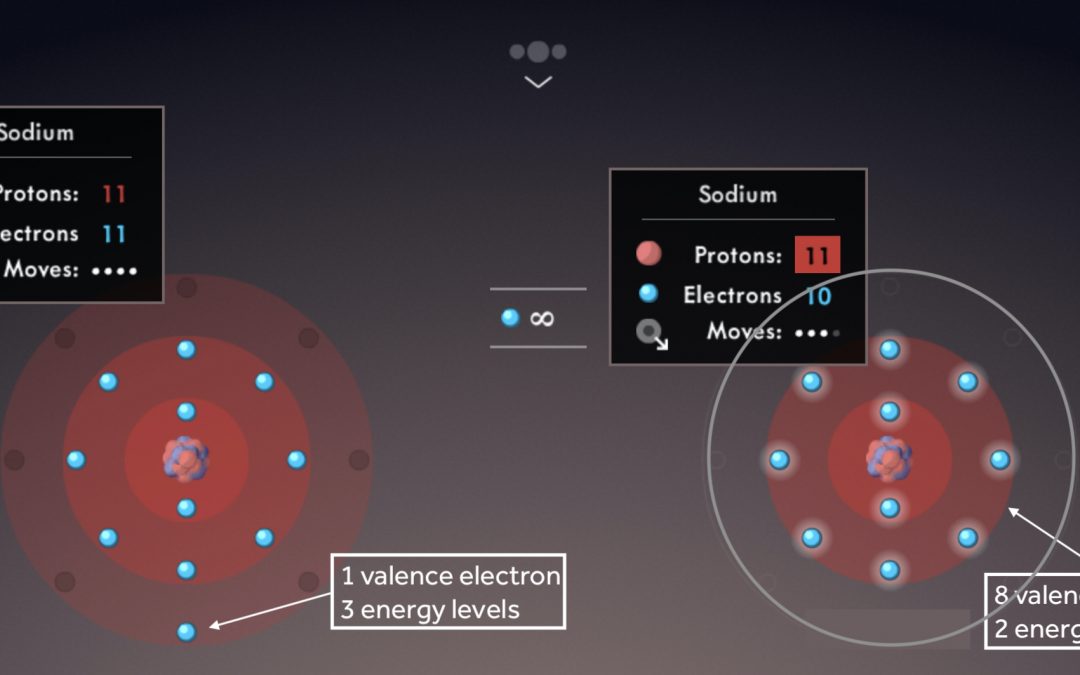
Illustrate the octet rule for cations by pointing out how the removal of one or more electrons uncovers a full shell underneath, which then becomes the valence shell.
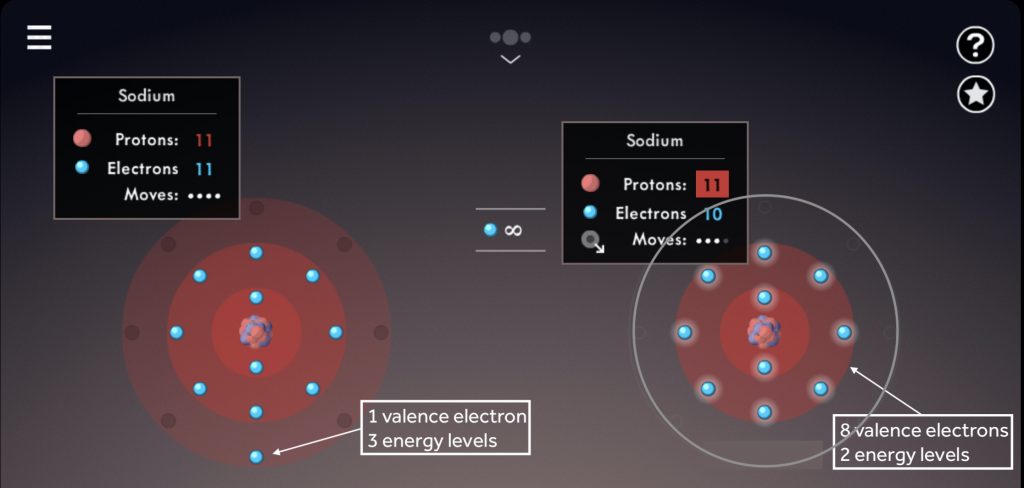
Note the number of valence electrons and energy levels within each sodium atom.
Draw students’ attention to the location of like-charged ions in the periodic table; ask students to predict ion charges based on element groups.
Explain the electrostatic force between protons and electrons that drives effective nuclear charge (Zeff), and how it affects the ionization energy. Post these short TikTok videos for your students to watch!
After playing Level 5, have your students describe the ionization energy trend that is represented down a group, and explain why this happens.
After playing Level 15, have your students describe the ionization energy trend that is represented across a period, and explain why this happens.
Have your students identify ionization energy trends, and draw the trends as big arrows across each period and down each group on a blank periodic table.
Challenge your students to master the Ions Sandbox Achievements:
- Cation and anion formation
- Specific ion charges
- Ionization energy trends
- Electron affinity trends
- Ionic radii
- Or, make up your own challenges and have students submit a screenshot of their work!
The Ions game shares Connected Levels with Atoms and Ionic Bonding. Have your students complete both the Ions and Atoms games, or Ions and Ionic Bonding, to open up the pipe between these games and CONNECT their learning!