Have you been using the Collisions: Phase Change Game with your students? Below are some additional strategies to help with planning your lessons.
- Before starting the game, ensure that students know the meaning of the following terms: solid, liquid, gas, phase change, energy.
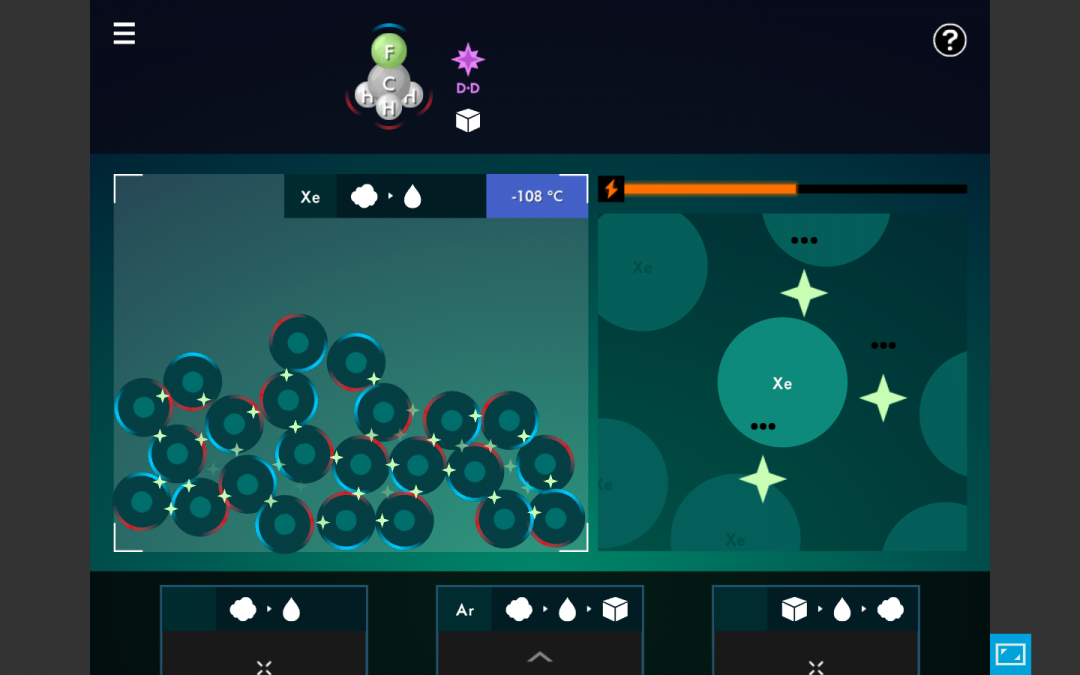
- As students play, introduce the terms latent heat, intermolecular forces, exothermic process, endothermic process.
- Illustrate that stronger intermolecular forces (denoted by the number of points on the “star”, as well as the number of clicks it takes to break them) have higher boiling points.
- Draw students’ attention to the temperature DURING a phase change – available energy is used to break the intermolecular forces, but the temperature does NOT rise. This is called “latent heat.”
- Explain how adding energy causes temperature rise or phase changes. Post these short YouTube videos for your students to watch!
Ask your students:
- After playing Level 6, describe which types of phase changes are exothermic.
- After playing Level 12, describe the relationship between IMFs and energy used.
- Which IMFs require more energy to break or form?
Challenge your students to master the Phase Change Sandbox Achievements:
- Six different types of phase changes
- Lower/Higher boiling points than a certain compound
- Exothermic vs endothermic phase changes
- Latent heat
- Or, make up your own challenges and have students submit a screenshot of their work!
The Phase Change game shares Connected Levels with IMFs and Ionic Bonding. Have your students complete both the Phase Change AND the IMFs (and/or) the Ionic Bonding game, to open up the pipe between these games and CONNECT their learning!